Electrolyte as basis for Ion-Lithium Batteries plays a key role in transporting the positive lithium ions between the cathode and anode, and consequently the charging and discharging performance of the battery. Hence, it needs to be checked for potential impurities. At the same time the electrolyte is also a sample type that allows the investigation of ageing processes, as degradation products from all components of the battery can accumulate within it over time.
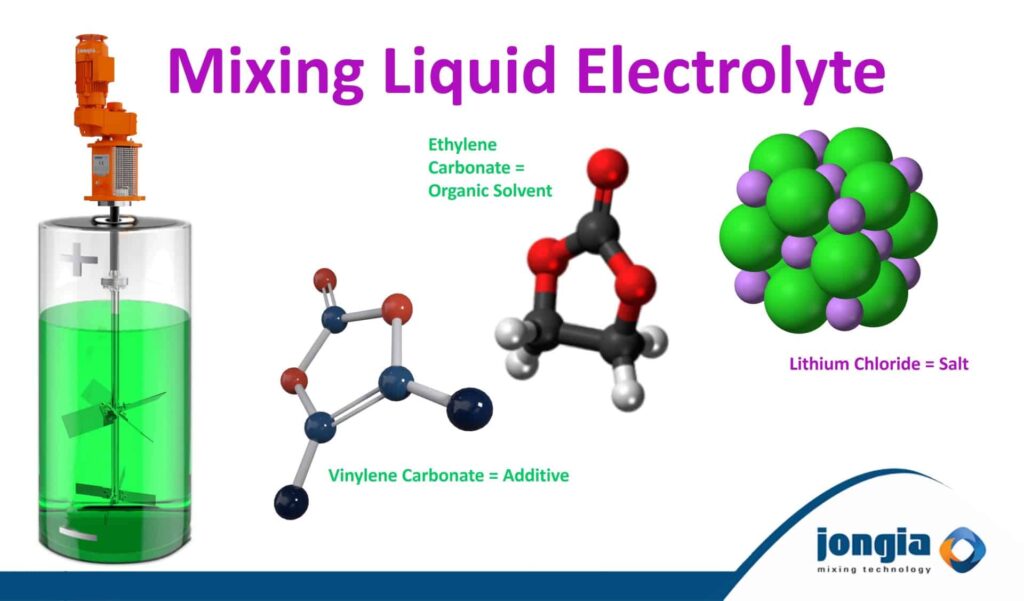
Electrolyte consists of a conducting salt in an organic solvent
The most common electrolyte salt is lithium hexafluorophosphate (LiPF6), but there are also lithium perchlorate (LiClO₄), lithium tetrafluoroborate (LiBF₄), lithium hexafluoroarsenate (LiAsF₆), lithium hexafluorosilicate (LiSiF₆), and lithium tetraphenylborate (LiB(C₆H₅)₄). Electrolyte in lithium-ion batteries is often a mixture of lithium salts and additional organic solvents.
Considering the chemical compound variety in the electrolyte (salts, ionic species, organic solvents, metals, etc.), different analytical techniques are required depending on analytical challenge. For instance, anions of lithium salts can be determined by ion chromatography (IC) to ensure that the solutions have been prepared at the proper concentrations. Gas chromatography or gas chromatography–mass spectrometry (GC-MS) can be used to provide the qualitative and quantitative composition of organic solvents in the electrolyte and aging byproducts.
Electrolyte plays a key role in transporting the positive lithium ions between the cathode and anode
The most commonly used electrolyte is comprised of lithium salt, such as LiPF6 in an organic solution.
Electrolyte is being applied as a liquid which demands a mixing process. This process is to ensure that the components of which Electrolyte is build up form, is properly blended. So, proper mixing with a suitable agitator type is of great importance!
The Electrolyte plays a pivotal role as one of the major four components of a battery
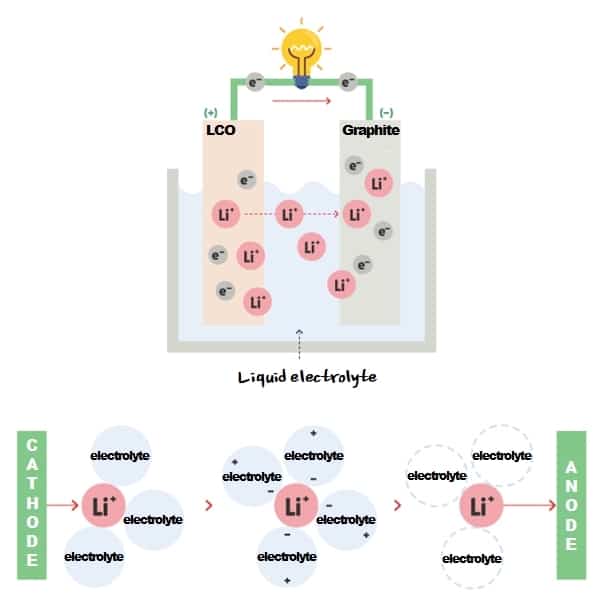
An Ion-lithium battery uses liquid electrolytes. It allows lithium ions(Li+) to move between anode and cathode, stabilizes cathode and anode surfaces, extends battery lifespan, and improves cell performances!
In a nutshell, liquid electrolytes serve as ‘a liquid that enables movement of lithium ions’ between cathode and anode. Electrolyte is the best driver that drives fast and safely for lithium ions.
Liquid electrolytes are consisted of lithium salt, organic solvent, and additives
The most commonly used lithium salt is LiPF6 (composed of lithium·phosphate·fluorine). LiPF6 offers better ion movement, dissolution, and higher chemical stability.
Lithium salt, passage of lithium ions
Lithium salt serves as a passage for lithium ions. So, lithium ions should be easily dissolved by means of proper mixing and dissociated in a solvent. These dissociated ions will then move smoothly, being properly mixed into the liquid.
Organic solvent, the liquid that dissolves lithium salt
Organic solvent will dissolve lithium salt when this is blended properly by an agitator and helps lithium ions travel easily. There are some requirements for organic solvents. Organic solvents should have high solubility for lithium salt to separate ionic compounds, and low viscosity for smooth movement of lithium ions.
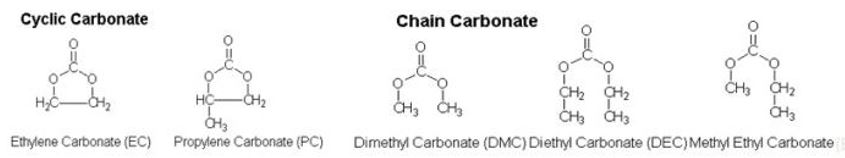
It varies by solvents, but in order to have high ionic conductivity, the combination of cyclic carbonate with high solubility for lithium salt should be considered, and chain carbonate with low viscosity.
Also a solvent should have low chemical reactivity. Lithium often responds to water, so the solvent of liquid electrolytes should not respond to water. Some organic solvents used in the electrolyte solution are ethylene carbonate, diethyl carbonate, dimethyl carbonate, ethyl methyl carbonate, propylene carbonate, methyl formate, methyl acrylate, methyl butylate and ethyl acetate.
And now we come to the additive;
The Additive, a substance that decides liquid electrolytes.
Additive, a substance which is added in small quantities, is forming a protective film on the Ion-Lithium Battery cathode and anode surfaces. It helps smooth movement of lithium ions between cathode and anode, and prevents battery degradation.
Additives are divided into two categories: The Cathode and anode additives. Cathode additives stabilize a cathode structure and protect the surface to prevent battery aging, thus reducing overheating and overcharging. Additives such as vinylene carbonate (VC) and fluoroethylene carbonate (FEC) are commonly added to the electrolyte to improve the overall performance of lithium-ion batteries. Vinylene carbonate is used widely as an electrolyte additive for lithium-ion batteries where it promotes the formation of an insoluble film between the electrolyte and the negative electrode: the SEI (solid-electrolyte-interface). This polymer film allows ionic conduction, but prevents the reduction of the electrolyte at the negative (graphite) electrode and contributes significantly to the long-term stability of lithium-ion batteries.
Anode additives are dissolved earlier than a solvent, creating a strong film in the anode to increase lifespan, prevent overheating and maintain battery capacity.
Both cathode and anode additives should be well dissolved in liquid electrolytes by means of a well selected agitator and chemically stable. Therefore, different additives are used according to the required specifications or different purposes.
Additives, however take only a small part of the liquid electrolyte. Still, they play a crucial role in the entire system by increasing lifespan, improving high-temperature issues, and reducing resistance.
Liquid electrolyte is ‘a medium that moves lithium ions fast and safely’, so it should be able to move ions smoothly. And it should be chemically electrically stable not to undermine battery performance.
Because in some cases, liquid electrolytes have side reaction while the battery is operated. The freezing point should be low and the boiling point should be high so that the battery can operate anytime.
Now, which type of agitation or mixing elements is the best choice to ensure that the ingredients for the electrolyte are properly mixed?
Jongia Mixing Technology has many experiences for selecting suitable mixers for the production of electrolyte. Since the viscosity of the components for Electrolyte are quite low and since the Lithium Chlorides should be avoided to sink, Jongia Mixing Technology will select an agitator with multi-stage mixing elements with axial mixing turbines. These turbines will create sufficient top-over bottom flow, without having a too high shear and consuming the lowest powerintake. If the Electrolyte tank is having a conical or spherical bottom shape, Jongia will add a rest-stirrer to ensure that all the components will be properly mixed throughout the whole volume of the electrolyte vessel.
What is of great importance to select the most optimum electrolyte mixer?
The Geometry of the electrolyte tank in combination with the deviation of the electrolyte components should be clear in order to select the most appropriate agitator.
Jongia Mixing Technology is always able to assist in the guidance of the selection for the agitator configuration. Just contact Jongia and ask for assistance.
We are always available for your mixer requests.
Contact us at: info@jongia.com or visit the Batteries page on our website for more Ion-Lithium battery related articles.
This article has been written by making use of certain information coming from the following websites: Samsungsdi.com and Wikipedia
Contact our specialized team for all your questions

Bart Brouwer
Area Sales Manager
Area Worldwide

Sijko van der Veen
Application Engineer
Technical Specialist
Technical Questions?

Jan Siert Tjeerdsma
Project Manager
Technical Specialist
Related Articles

Is Sodium-Ion the next generation sustainable Battery?
The sodium-ion battery (NIB or SIB) is a type of rechargeable battery that uses sodium ions (Na+) as its charge carriers. Its working principle and cell construction are almost identical with those of lithium-ion battery (LIB) types, but replace lithium with sodium.

What is the recycling process for lithium
Current commercial lithium ion batteries mainly contain transition metal oxides or phosphates, aluminum, copper, graphite, organic electrolytes containing harmful lithium salts, and other chemicals. Therefore, the recycling and reuse of spent lithium ion batteries has been paid more and more
Mixing the correct Ion-Lithium Battery Slurry is a real challenge!
Lithium-based battery technologies for electric vehicles use lithium-ions as the charge carrier. Depending on the application’s technical requirements, lithium is used with various chemistries such as graphite for the anode as well as nickel, manganese or cobalt oxides for the